Extended use or re-use of single-use surgical masks and filtering facepiece respirators: A rapid evidence review
June 5, 2020

Elaine Toomey1, Yvonne Conway2, Chris Burton3, Simon Smith4, Michael Smalle5,
Xin-Hui Chan 6, Anil Adisesh7, Sarah Tanveer8, Lawrence Ross9, Iain Thomson10,
Declan Devane2, Trisha Greenhalgh11
1School of Allied Health, University of Limerick, Limerick
2School of Nursing and Midwifery, National University of Ireland, Galway
3Academic Unit of Primary Medical Care, University of Sheffield
4Canadian Standards Biological Aerosols Working Group
5James Hardiman Library, National University of Ireland, Galway
6Nuffield Department of Medicine, University of Oxford
7University of Toronto and St. Michael’s Hospital, Unity Health, Toronto
8Department of Pharmaceutical Health Services Research, University of Maryland, Baltimore
9Department of Infectious Disease, Children’s Hospital of Los Angeles
10Médecins Sans Frontières/Doctors without Borders
11Nuffield Department of Primary Care Health Sciences, University of Oxford
Correspondence to elaine.c.toomey@ul.ie
VERDICT
This review synthesises and compares international guidance and systematic review evidence on extended use, re-use or reprocessing of single-use surgical mask and filtering facepiece respirators.
The main findings were:
- While extended use or re-use of single-use surgical masks or respirators (with or without reprocessing) is generally not recommended, guidance from various organisations supports such measures (preferably extended use rather than re-use) as a last-resort measure during critical shortage.
- Comparisons across guidance documents and systematic reviews highlight limited evidence, varying levels of detail, and areas of inconsistency, especially in relation to re-use of respirators (with or without reprocessing) during and after aerosol generating procedures.
- The reprocessing of surgical masks is not recommended.
- Reprocessing of respirators under controlled and standardised conditions is recommended, but there is inconsistency regarding how or when this should take place and further research is needed in this area.
- Where extended use or re-use is being practised, healthcare facilities and institutions should ensure that policies and systems are in place to enable these practices to be carried out in the safest way possible in line with available guidance.
A full-text paper has been submitted to an academic journal and a pre-print with supplementary files has been submitted to medRxiv. The review protocol and associated materials are available at https://osf.io/7c6rs/
BACKGROUND
The COVID-19 pandemic has led to unprecedented demand for personal protective equipment. Shortages of surgical masks and filtering facepiece respirators has led to the extended use or re-use of single-use respirators and surgical masks by frontline healthcare workers, however the evidence base underpinning these practices is unclear. A number of national and international guidelines make reference to extended use, re-use and reprocessing of single-use masks and respirators1. We compared these guidelines first with each other and second with current synthesised evidence, particularly in the light of current worldwide shortages of personal protective equipment, in order to inform rapidly evolving policies and practice. The review was conducted in line with Cochrane rapid review interim guidance2.
IDENTIFICATION AND SYNTHESIS OF STUDIES
A targeted search of the World Health Organization, European Centre for Disease Prevention and Control, the US Centers for Disease Control and Prevention, and Public Health England websites was conducted to identify guidance. Four databases (Medline, Pubmed, Epistemonikos, Cochrane Database of Systematic Reviews) and three preprint repositories (Litcovid, MedRxiv and Open Science Framework) were searched for relevant systematic reviews. A full search strategy is provided below.
We defined extended use as the practice of using the same single-use mask or respirator for encounters with multiple patients, without removing it3. Re-use was defined as the practice of using the same mask or respirator for multiple encounters with patients, removing it (‘doffing’) for storage after each encounter and putting it on again (‘donning’) prior to the next encounter with a patient3. Reprocessing was defined as ‘decontamination using disinfection or sterilization methods followed by re-use of either reusable or disposable PPE’4. Record screening and data extraction was conducted by two reviewers. Quality of included systematic reviews was appraised by two reviewers using the AMSTAR-2 checklist. Findings were integrated and narratively synthesised.
SUMMARY OF FINDINGS
Six documents (US Centers for Disease Control and Prevention3 5 6, the European Centre for Disease Prevention and Control7, Public Health England8, and the World Health Organization4) were identified. All were published or updated between March 17th 2020 and May 21st 2020 and written for the COVID-19 pandemic.
Four relevant systematic reviews were included9-12. Three focused on reprocessing of filtering facepiece respirators using different decontamination interventions,9-11 and one covered reprocessing of surgical masks and ‘pre-contamination’ interventions applied before use to enable extended use or re-use. Included systematic reviews were judged to be predominantly of high quality using AMSTAR 2. No reviews in our sample examined the impact of extended use or re-use of filtering facepiece respirators or surgical masks on the ability to meet technical standards or on healthcare worker acceptability outcomes such as comfort. Only two studies13 14 included in one review11 explored the effect of reprocessing on SARS-CoV-2.
Figure 1 provides a schematic summary of current international guidelines on re-use, reprocessing, and extended use of single-use masks and respirators. While extended use or re-use of single-use surgical masks or respirators (with or without reprocessing) is generally not recommended, guidance from various organisations supports such measures (preferably extended use rather than re-use) as a last-resort measure during critical shortage. Tables 1-2 summarise guidance on extended use, re-use and reprocessing of surgical masks and respirators respectively. Table 3 compares the findings of the systematic reviews with the three guidance documents relating to reprocessing of surgical masks and respirators. Comparisons across guidance documents and systematic reviews highlight limited evidence, varying levels of detail, and areas of inconsistency, especially in relation to re-use of respirators (with or without reprocessing) during and after aerosol generating procedures. Reprocessing of respirators under controlled and standardised conditions is recommended, but there is inconsistency regarding how or when this should take place.
CONCLUSIONS
Extended use and re-use of single-use surgical masks and respirators (with or without reprocessing) should only be considered in situations of critical shortage. Where extended use or re-use is being practiced, healthcare organisations should ensure that policies and systems are in place to ensure these practices are carried out as safely as possible and in line with available guidance. Areas of guidance lacking clarity and consistency warrant further attention and investigation.
Figure 1: Schematic summary of current international guidelines on re-use, reprocessing, and extended use of single-use masks and respirators
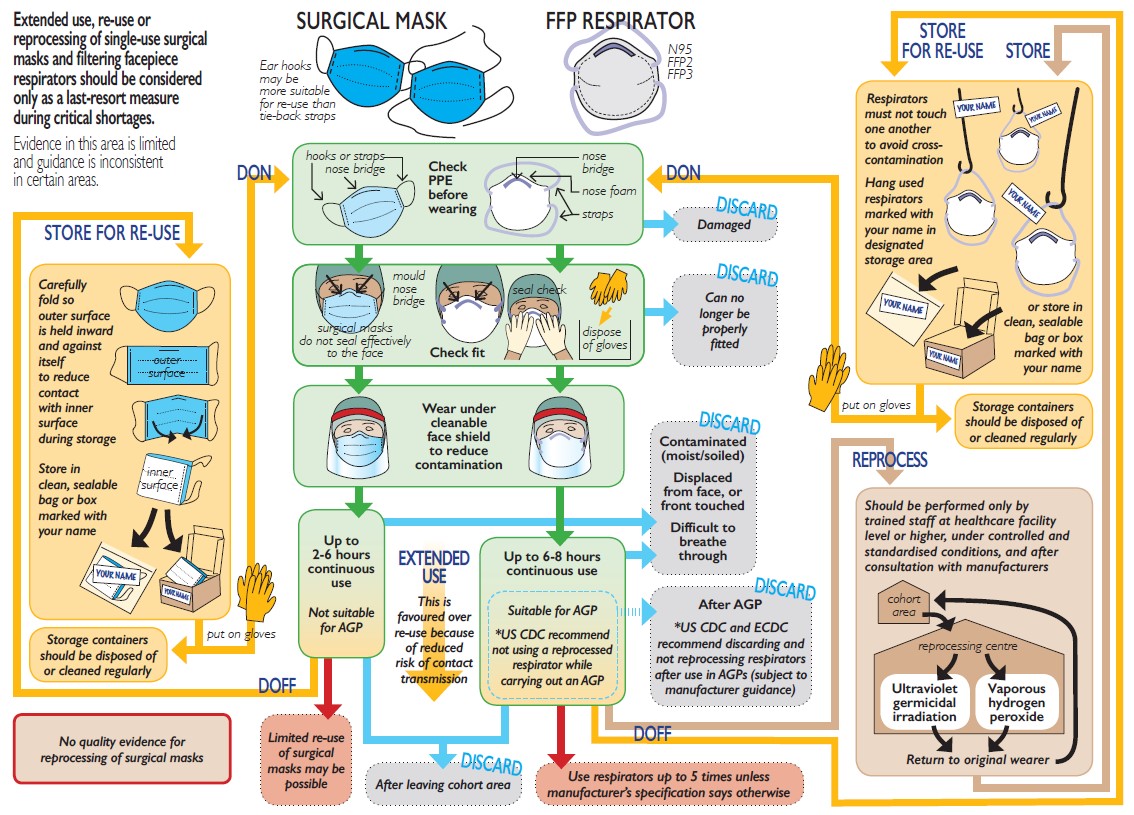
Table 1 Summary of guidance recommendations for fluid resistant surgical masks
|
CDC |
ECDC |
PHE1 |
WHO |
| Extended use |
| Discarding |
Soiled, damaged or hard to breathe through |
No information |
Moist, damaged, visibly soiled or difficult to breathe through. |
Wet, soiled, splashed, damaged or difficult to breathe through.
If displaced from face or touched |
| Maximum duration |
Not specified |
2-6 hours dependent on setting and activity |
Up to 6hrs
|
| Safe use |
Hand hygiene if adjusted/ touched
Only continue within cohorted area. |
Hand hygiene if adjusted/ touched
Discard after leaving cohorted area unless transferring a patient |
Only continue within cohorted area – discard on leaving |
| Re-use |
| Discarding |
Soiled, damaged or hard to breathe through |
Not recommended |
Moist, damaged, visibly soiled or difficult to breathe through. |
No information |
| Maximum uses |
Not specified |
Not specified |
| Safe use |
Do not re-use masks that fasten with ties (elastic ear hooks more suitable)
Fold2 carefully and store in sealable paper bag or breathable container between uses. |
Masks with elastic ear hooks more suitable for re-use
Fold2 carefully and store in labelled sealable bag between uses
Some models cannot be re-used as they deform once being donned |
| Reprocessing |
| Discarding |
Not recommended |
| Maximum duration / uses |
| Safe use |
1 PHE guidelines on extended use and re-use do not clearly differentiate between surgical masks and filtering facepiece respirators
2 “Fold” – this cannot apply to moulded cup-type face masks
Table 2 Summary of guidance recommendations for filtering facepiece respirators
|
CDC |
ECDC |
PHE3 |
WHO |
| Extended use |
| Discarding |
Soiled (blood, secretions, body fluids), if obvious damage or difficult to breathe through
Discard after use in aerosol generating procedure.
|
Soiled, wet, can no longer be properly fitted or difficult to breathe through
Discard after use in aerosol generating procedure |
Moist, damaged, visibly soiled or difficult to breathe through. |
Wet, soiled, splashed, damaged or difficult to breathe through.
If displaced from face or touched |
| Maximum duration |
Not specified (notes some studies up to 8hrs) – guided by hygienic/practical concerns |
Time guided by hygienic/practical concerns4 |
Not specified5 |
Up to 6 hours |
| Safe use |
Hand hygiene if adjusted or touched. Respirator can be covered with face shield (‘strongly preferred’) or surgical mask. Discard after leaving cohorted area. |
Respirator can be covered with face shield or medical mask to extend use
|
Hand hygiene if adjusted or touched. Discard after leaving cohorted area unless transferring a patient |
Only continue within cohorted area – discard after leaving |
| Re-use6 |
| Discarding |
Soiled (blood, secretions, body fluids), if obvious damage or difficult to breathe through
Discard after use in aerosol generating procedure. |
No information |
Moist, damaged, visibly soiled or difficult to breathe through. |
Re-use without re-processing strongly discouraged |
| Maximum uses |
Up to 5 times unless manufacturer states otherwise. |
Not specified |
| Safe use |
(a) Ensure safe storage and limit to named user
(b) Follow one day of use with 4 day “quarantine” period in labelled breathable sealed container before re-use7 |
Fold and store in labelled sealable bag between uses
Some models cannot be re-used as they deform once being donned |
| Reprocessing4 |
| Discarding |
If integrity of any component (e.g. straps, bridge) of the respirator is compromised, or if a successful user seal check cannot be performed. |
Not specified |
Currently no recommendations (but acknowledge work on validating methods in progress) |
Check integrity and shape before reprocessing – discard if damaged/not suitable for re-use |
| Maximum duration / uses |
Not specified |
Not specified |
“After a pre-defined number of uses” |
| Safe use |
Not for use in aerosol generating procedures8 |
Quality checks of reprocessing necessary to ensure safety |
After use, label and place in reprocessing container Reprocessing performed by trained staff
Return to original wearer after reprocessing |
1 PHE guidelines on extended use and re-use do not clearly differentiate between surgical masks and filtering facepiece respirators
2 “..can be re-used for a limited time, unless there is a risk for contamination through the deposition of infectious particles on the surface”
3 States that filter capacity of FFP3/FFP2/N95 respirators greatly exceeds one day of use in health/social care
4 Re-used and reprocessed respirator are likely to be used for extended periods, in this case the extended use criteria also apply.
5 Point (b) currently appears in decontamination guidelines as an alternative to decontamination, but does not appear in re-use guideline.
6 unless manufacturer information indicates that decontamination does not affect performance
Table 3 Summary of systematic review conclusions compared to guidance recommendations for reprocessing methods
| Component* |
Type |
Method |
Review |
Review conclusion |
CDC |
ECDC |
WHO |
| Filtering facepiece respirators |
Microwave & heat based treatments |
Microwave (dry / moist) 60-90ºC |
Gertsman 2020 |
Effective sterilisation while maintaining mask integrity (some models) |
Moist heat is promising**
Steam treatment is promising with some limitations – variability of microwaves used to generate steam, sparking concerns. Dry microwave irradiation/dry heat not recommended. |
Not mentioned |
Not recommended – inconsistency of machines; overheating/ sparking of metal nose band |
| Other heat (dry / moist) 60-90ºC
|
Effective sterilisation while maintaining mask integrity (some models) |
Steam sterilisation at temperatures <134 oC under study |
Steam sterilisation at 134oC not recommended
|
| Autoclaving/heat>90ºC |
Not recommended – damage to mask integrity |
Not recommended |
Not recommended |
| Chemical disinfectants |
Vapourised hydrogen peroxide |
O’Hearn 2020a |
Removes pathogens without affecting function or fit; minimal impact on appearance |
Promising** |
Cautiously cites supportive studies – concern about residual chemicals and filtration |
Cautious support – limited number of models tested |
| Liquid hydrogen peroxide |
Further research needed on decontamination effects and impact on fit |
Promising with some limitations |
Not mentioned |
Not mentioned |
| Sodium hypochlorite |
Not recommended – adverse effect on function |
Not recommended |
Not mentioned |
Not recommended |
| Ethylene oxide |
Not recommended – potential health risk |
Not recommended – potentially harmful |
Mentioned but no recommendation made |
Cautious support – limited number of models tested |
| Ethanol/isopropyl alcohol |
Not recommended |
Not recommended |
Not mentioned |
Not recommended |
| Other methods |
Ultraviolet germicidal irradiation |
O’Hearn 2020b |
Effective decontamination of mask surfaces while maintaining mask integrity (some models) |
Promising**
Efficacy dose dependent
Proper precautions are required to avoid UVGI exposure to skin or eyes |
Mentioned but no recommendation made |
Cautious support – concerns about penetration of UV light to deeper layers of filter. |
| Disinfectant wipes |
– |
No systematic review evidence |
Not recommended |
Not mentioned |
Not mentioned |
| Gamma irradiation |
– |
No systematic review evidence |
Not mentioned |
Cautiously cites ongoing studies – concerns about availability, impact on fit |
No evidence in masks/respirators |
| Ozone decontamination |
– |
No systematic review evidence |
Not mentioned |
Mentioned but no recommendation made |
Not mentioned |
| Surgical masks |
All methods |
Dry and moist heat, ethanol, isopropanol, sodium hypochlorite (single studies only) |
Zorko 2020 |
Inadequate evidence on the safety or efficacy of any method
|
Not recommended |
Not recommended |
Not recommended |
Disclaimer: the article has not been peer-reviewed; it should not replace individual clinical judgement and the sources cited should be checked. The views expressed in this commentary represent the views of the authors and not necessarily those of the host institution, the NHS, the NIHR, or the Department of Health and social Care. The views are not a substitute for professional medical advice.
SEARCH TERMS (strategy used in Ovid MEDLINE)
1 exp Masks/
2 exp Respiratory Protective Devices/
3 (respiratory protective devices or mask* or face mask* or facemask* or respiratory protection or respirator* or FFP3 or FFP or N95 or N 95 or PAPR or air purifying respirator or filtering face piece).tw,kf.
4 (filtering adj3 (facepiece* or face piece*)).tw,kf.
5 or/1-4
6 Equipment Contamination/
7 exp Infection Control/
8 (infection control* or decontaminat* or resanitiz* or resanitis* or desaniti* or contaminat* or antisept* or biocid* or steriliz* or sanitize* or bleach* or hypochlor* or ozon* or ultraviolet or uv).tw,kf.
9 Sodium Hypochlorite/
10 Disinfectants/
11 Hydrogen Peroxide/
12 (clorox or antiformin or oxygenated water or hydrogen peroxyde or hydroperoxide or peroxygen or sodium hypochlorite).tw,kf.
13 Ethanol/
14 2-Propanol/
15 Ethylene Oxide/
16 (ethanol* or isopropanol or iso-propanol or 2-propanol or isopropyl alcohol or ethylene oxid* or oxirane or rubbing alcohol*).tw,kf.
17 or/6-16
18 (washable or rewash* or reusable or reprocess* or reus* or reusing or repurpose or recycl* or multiple use* or multiple usage or used again or used repeat* or repeat* use* or repeat* usag* or repeatedly use* or extended usage).tw,kf.
19 5 and 17 and 18
REFERENCES
- Kobayashi LM, Marins BR, Costa PCDS, et al. Extended use or reuse of N95 respirators during COVID-19 pandemic: An overview of national regulatory authority recommendations. Infect Control Hosp Epidemiol 2020:1-3. doi: 10.1017/ice.2020.173
- Garritty C, Gartlehner G, Kamel C, et al. Cochrane Rapid Reviews Interim Guidance from the Cochrane Rapid Reviews Methods Group 2020, 2020.
- US Centers for Disease Control and Prevention. Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings-NIOSH Workplace Safety and Health Topic 2020 [updated March 27, 2020. Available from: https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html.
- World Health Organization. Rational use of personal protective equipment for coronavirus disease ( COVID-19) and considerations during severe shortages: interim guidance, 6 April 2020: World Health Organization; 2020 [Available from: https://www.who.int/publications-detail/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages.
- US Centers for Disease Control and Prevention. Strategies for optimizing the supply of facemasks: CDC Atlanta, GA; 2020 [Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html.
- US Centers for Disease Control and Prevention. Decontamination and reuse of filtering facepiece respirators 2020 [Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html9.
- European Centre for Disease Prevention and Control. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators 2020 [Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Cloth-face-masks-in-case-shortage-surgical-masks-respirators2020-03-26.pdf.
- Public Health England. Considerations for acute personal protective equipment (PPE) shortages 2020 [updated April 27, 2020. Available from: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control/managing-shortages-in-personal-protective-equipment-ppe.
- Gertsman S, Agarwal A, O’Hearn K, et al. Microwave-and Heat-Based Decontamination of N95 Filtering Facepiece Respirators (FFR): A Systematic Review. 2020
- O’Hearn K, Gertsman S, Sampson M, et al. Decontaminating N95 masks with Ultraviolet Germicidal Irradiation (UVGI) does not impair mask efficacy and safety: A Systematic Review. 2020
- O’Hearn K, Gertsman S, Webster R, et al. Efficacy and Safety of Disinfectants for Decontamination of N95 and SN95 Filtering Facepiece Respirators: A Systematic Review. 2020
- Zorko D, Choong K, McNally JD, et al. Decontamination Interventions for the Reuse of Surgical Mask Personal Protective Equipment (PPE): A Protocol for a Systematic Review. 2020
- Fischer R, Morris DH, van Doremalen N, et al. Assessment of N95 respirator decontamination and re-use for SARS-CoV-2. medRxiv 2020:2020.04.11.20062018. doi: 10.1101/2020.04.11.20062018
- Kumar A, Kasloff SB, Leung A, et al. N95 Mask Decontamination using Standard Hospital Sterilization Technologies. medRxiv 2020:2020.04.05.20049346. doi: 10.1101/2020.04.05.20049346

