Rapidly managing pneumonia in older people during a pandemic
March 16, 2020
Pneumonia treatment in the elderly (2)PDF to download
Carl Heneghan, Jeff Aronson, Richard Hobbs, Kamal Mahtani
Updated 20th March: This article has been corrected
Please Check NICE guidance for all prescribing recommendations. (see the end of the article for an explanation)
3rd April: NICE guidance updated
COVID-19 rapid guideline: managing suspected or confirmed pneumonia in adults in the community
For general advice on managing COVID-19 symptoms, see also the NICE COVID-19 rapid guideline on managing symptoms (including at the end of life) in the community.
Rationale
The current COVID-19 pandemic has highlighted the risk faced by older adults, who are more susceptible to complications, including acute respiratory distress syndrome, usually as a result of pneumonia. Comorbidities, impaired immunity and frailty, including a reduced ability to cough and to clear secretions from the lungs, can all contribute to this complication. Older people are therefore more likely to develop severe pneumonia, suffer from respiratory failure, and die.
Viruses are thought to cause about 50% of cases of pneumonia. Viral pneumonia is generally less severe than bacterial pneumonia but can act as a precursor to it. Preventing any pneumonia in older adults is preferable to treating it.
Identification of the early stages of pneumonia in older patients can prove difficult. Traditional symptoms and signs, including fever, may be absent. Limited evidence suggests that many tests that are useful in younger patients do not help diagnose infections in older adults. The onset of pneumonia in elderly people can often be rapid, and the prognosis is poor in severe pneumonia: as many as one in five will die. The older you are, the more prevalent severe pneumonia becomes.
Patients in nursing homes appear to fare even worse, as they often have several comorbidities and poor nutritional status and are often physically inactive. [5] In-hospital mortality is significantly higher, even after adjusting for age and sex.
Common causative organisms in elderly people admitted to hospital with pneumonia include Streptococcus pneumoniae and Mycoplasma pneumoniae. Less commonly, Haemophilus influenzae and Staphylococcus aureus may be responsible. In severe pneumonia, S. aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa are common causative organisms. In community acquired pneumonia, the causative agent is often not known.
Older patients may have polymicrobial infections, which could be a factor in those who do not respond to initial antimicrobial treatment. Sputum cultures are often not reliable, as the microbial aetiology of severe pneumonia based on invasive diagnostic techniques often differs from the organisms found in the sputum.
Assessment of 12,945 US Medicare in-patients with pneumonia, aged over 65, showed that initial treatment with a second-generation cephalosporin plus a macrolide, or a non-antipseudomonal third-generation cephalosporin plus a macrolide, or a fluoroquinolone alone lowered 30-day mortality. And an analysis of 101 patients aged > 75 (mean and SD, 82 ± 5.5) admitted to an intensive care unit reported significantly higher mortality in those who received inadequate antimicrobial therapy (39% versus 4%; P = 0.007).
Viral infections increase pneumococcal adherence to the local epithelium, facilitating bacterial infection. Adhesion of Streptococcus pneumoniae to epithelial cells, for example, is significantly enhanced by human coronavirus HCoV-NL63 infection. Coronavirus causes inflammatory damage in the lungs, preventing clearance of bacteria. Secondary bacterial infection worsens prognosis. Most deaths in the influenza pandemics of 1918, 1957, and 1968 were caused by secondary bacterial infections. Concurrent bacterial pneumonia was highlighted as a particular problem in elderly people in the 2003 SARS outbreak.
Early use of antibiotics in older adults
Non-response to initial antimicrobial therapy increases mortality, and so the initial selection of antimicrobials is critical. According to NICE, to cover atypical and multiple pathogens in older patients with pneumonia and at risk of severe complications, the recommended choices of antibiotics in the community are:
| Amoxicillin with |
500 mg 3 times a day (higher doses can be used – see BNF) for 5 days |
| Clarithromycin (if atypical pathogens) |
500 mg twice a day for 5 days |
| Alternative oral antibiotics for penicillin allergy, if the pneumonia is of moderate-intensity; treatment should be guided by microbiological results when available |
| Doxycycline or |
200 mg on the first day, then 100 mg once a day for a further 4 days (5‑day course in total) |
| Clarithromycin |
500 mg twice a day for 5 days |
Please note there was an error with the prescribing strategy and this has been corrected as of 20th March –
Please Check NICE guidance for all recommendations. ‘
Prescribe oral amoxicillin 500 mg three times a day for 5 days (higher doses can be used — see the BNF) and (if atypical pathogens suspected) oral clarithromycin 500 mg twice a day for 5 days, or oral erythromycin (in pregnancy) 500 mg four times a day for 5 days. Alternatively, if there is a penicillin allergy, or amoxicillin is unsuitable (for example atypical pathogens are suspected) options are oral doxycycline 200 mg on the first day then 100 mg once a day for 4 days (total course of 5 days), or oral clarithromycin 500 mg twice a day for 5 days, or oral erythromycin (in pregnancy) 500 mg four times a day for 5 days.’
The intensity of pneumonia in the community can be assessed using the CRB65 score; each factor scores one point:
- confusion (abbreviated Mental Test score 8 or less, or new disorientation in person, place, or time);
- a raised respiratory rate (30 breaths per minute or more);
- a low blood pressure (diastolic 60 mmHg or less, or systolic less than 90 mmHg);
- age 65 years or over.
Score 1 or 2: intermediate risk (1‑10% mortality risk).
Score 3 or 4: high risk (more than 10% mortality risk).
NICE recommends that anyone with a score of 2 should be admitted to hospital. NICE’s approach, however, doesn’t account for the high risk in very elderly people. The mortality rate from COVID-19 approaches 15% at age 80 (Figure 1).
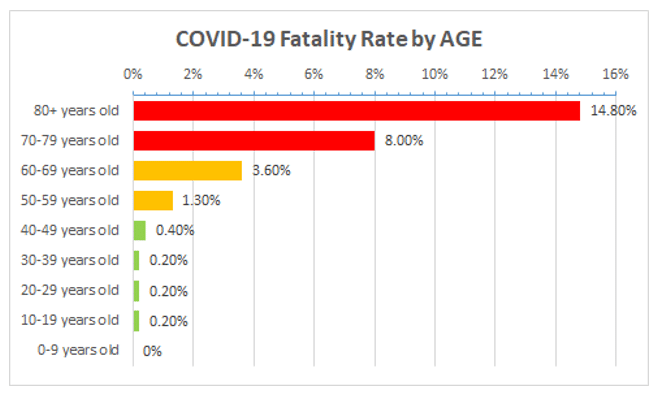
Source data: https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/ (accessed 13 March 2020)
Current NICE guidance requires starting antibiotic treatment as soon as possible after establishing a diagnosis of community-acquired pneumonia, and certainly within 4 hours. This strategy is supported by the results of a US multicenter retrospective cohort study, a medical record review of 14,069 patients aged over 65 years and hospitalized with pneumonia. A lower 30-day mortality was associated with antibiotic administration within 8 hours of hospital arrival (odds ratio = 0.85; 95% CI = 0.75-0.96).
However, this may not be possible within the constraints of an overstretched service in a pandemic. If antibiotics have to be taken within 4 hours of onset, there needs to be in place a self-management strategy that permits rapid access to the right antimicrobial treatment. Nursing homes could hold stocks of antibiotics for rapid deployment. Health professional confirmation could then be used to facilitate timely self-prescribing for those most at risk.
Examination strategy
The least amount of equipment that is clinically appropriate should be used to assess a patient who might have COVID-19. This should include a pulse oximeter, a thermometer, and a stethoscope. The ‘eyeball’ test, incorporating information on temperature, oxygen saturation, and pulse rate, should be sufficient to assess severity and cut down significantly on contact time. The absence of any individual chest examination finding has little effect on the probability of diagnosing pneumonia. Assessing blood pressure significantly increases contact time and should be considered only in those in whom it contributes to the decision to admit or not. We recommend documenting that a ‘limited examination’ was performed.
In assessing patients, carry antibiotics in a pre-sealed bag, to cut down entry and exit times from the person’s home or nursing home.
COVID Monitoring Service (CMS)
Patients at high risk deemed to be managed at home require monitoring, to ensure that they do not deteriorate. This is essential for nursing homes, where the potential for further spread in their patient population is significant. Telephone monitoring services can follow up patients to determine whether deterioration occurs, and to detect spread in nursing homes.
Based on evolving NHS England guidance we recommend the following pathways
Category 1: May require admission
When it is uncertain that safe care can be provided in the community and the patient is deteriorating.
Clinical pathway: Start antibiotics immediately; discuss management with a designated hospital admitting consultant.
Category 2: Home isolation with active health monitoring
Higher risk group for severe COVID-19, with stable illness that can be managed in the community.
Clinical pathway: Signs of bacterial pneumonia–start antibiotics; active health monitoring (every other day calls and symptom monitoring) and point of contact if deteriorates. Nursing homes with active cases–daily calls to monitor individuals and case progression; patients should remain in isolation until 5 days after resolution of symptoms.
Category 3: Home isolation with health advice
Mild illness in a patient who can be managed safely in the community AND who is not in a higher risk group for complications.
Clinical pathway: Self isolate at home; health advice on how to identify deterioration; call CMS if more unwell.
Gov.UK on those at increased risk of severe illness from coronavirus (COVID-19) includes:
- aged 70 or older (regardless of medical conditions)
- under 70 with an underlying health condition listed below (ie anyone instructed to get a flu jab as an adult each year on medical grounds):
- chronic (long-term) respiratory diseases, such as asthma, chronic obstructive pulmonary disease (COPD), emphysema or bronchitis
- chronic heart disease, such as heart failure
- chronic kidney disease
- chronic liver disease, such as hepatitis
- chronic neurological conditions, such as Parkinson’s disease, motor neurone disease, multiple sclerosis (MS), a learning disability or cerebral palsy
- diabetes
- problems with your spleen – for example, sickle cell disease or if you have had your spleen removed
- a weakened immune system as the result of conditions such as HIV and AIDS, or medicines such as steroid tablets or chemotherapy
- being seriously overweight (a body mass index (BMI) of 40 or above)
- those who are pregnant
Oxygen
The use of oxygen in pneumonia is based on expert opinion. A Cochrane systematic review of the effectiveness of oxygen in adults with pneumonia highlights that the evidence is weak and limited owing to a small number of studies. The British Thoracic Society suggests that for patients with pneumonia not at risk of hypercapnic respiratory failure, it is appropriate to aim for an oxygen saturation of 94–98%. Very elderly patients may tolerate an abnormally low SaO2 at rest when clinically stable; however, COVID-19 pneumonia may significantly worsen SaO2. Access to oxygen therapy will be challenging at the height of a pandemic.
Corticosteroids
In the initial phase of pneumonia, elderly patients can present with wheezing and respiratory distress. It is not uncommon to consider corticosteroids at this stage, because of their anti-inflammatory effects. Corticosteroids were widely used during the 2002-3 SARS outbreak. However, in a subsequent systematic review, including 29 low-quality studies of steroid use, 25 studies were inconclusive and four reported possible harm from steroid use. A further evidence review did not support corticosteroid treatment, reporting no evidence of net benefit with corticosteroids in “respiratory infection due to RSV, influenza, SARS-CoV, or MERS-CoV”, and that corticosteroids probably impair clearance of SARS-CoV. In contrast to pneumonia, corticosteroids show much clearer benefit in patients with sepsis.
Potential harms of rapid deployment of antibiotics for pneumonia
The main disadvantage of this proposed strategy is that it would tend to drive increased bacterial resistance. However, in a pandemic with a high mortality rate in a specific subpopulation, in this case very elderly people, this needs to be weighed against the benefits of the policy. Apart from penicillin allergy, adverse reactions to the recommended antibiotics, e.g. macrolides, are generally mild and uncommon.
Conclusions
Interventions that affect mortality in pneumonia are of great significance for public health, particularly during the current pandemic. Rescue prescribing strategies, initiated by the patient at an early stage, could aid effective delivery of antimicrobials, significantly reduce hospital admissions, and reduce mortality. While reducing antimicrobial resistance should remain a global priority, the current pandemic highlights the need for unprecedented management strategies. For example, in the current context, it may be entirely appropriate for nursing homes to have routine stockpiles of antibiotics, allowing rapid and appropriate prescribing decisions that could minimize morbidity and mortality, as well as reducing the impact of the pandemic on health services. Rapid interventions like this could be life-saving.
A UK strategy of suppression including 14-day isolation for households with symptoms. In this context, equipping patients with rescue antibiotics may be a legitimate strategy to consider. Strategies should also plan to ensure that all those eligible at risk, eg COPD and immuno-compromised as well as the elderly, were up to date with preventive immunisation (influenza and pneumococcal). And also consider rescue medication for those aged 70 or older (regardless of medical conditions), and under 70 with an underlying health condition instructed to get a flu jab as an adult each year on medical grounds.
In patients with few physiological reserves, there is no room for error, and providing the right initial treatment, and rapidly, matters.
Disclaimer: the article has not been peer-reviewed; it should not replace individual clinical judgement and the sources cited should be checked. The views expressed in this commentary represent the views of the authors and not necessarily those of the host institution, the NHS, the NIHR, or the Department of Health. The views are not a substitute for professional medical advice. Check NICE guidance for the prescribing strategy
We welcome feedback on this page and if there are errors or omissions then please let us know at cebm@phc.ox.ac.uk
CORRECTION 20th March 2020 made.
Please note there was an error with the prescribing strategy the original version stated doxycycline plus clarithromycin, which is incorrect. The article should have stated doxycycline or clarithromycin as per NICE recommendations.
Please Check NICE guidance for all recommendations. ‘Alternatively, if there is a penicillin allergy, or amoxicillin is unsuitable (for example atypical pathogens are suspected) options are oral doxycycline 200 mg on the first day then 100 mg once a day for 4 days (total course of 5 days), or oral clarithromycin 500 mg twice a day for 5 days, or oral erythromycin (in pregnancy) 500 mg four times a day for 5 days.’
Authors
 Carl Heneghan is the Editor in Chief BMJ EBM, Professor of Evidence-Based Medicine, Director of the Centre for Evidence-Based Medicine and Director of Studies for the Evidence-Based Health Care Programme. (Full bio and disclosure statement here)
Carl Heneghan is the Editor in Chief BMJ EBM, Professor of Evidence-Based Medicine, Director of the Centre for Evidence-Based Medicine and Director of Studies for the Evidence-Based Health Care Programme. (Full bio and disclosure statement here)
 Jeffrey K. Aronson is a physician and clinical pharmacologist working in the Centre for Evidence-Based Medicine in the Nuffield Department of Primary Care Health Sciences, University of Oxford. He is an Associate Editor of BMJ EBM and a President Emeritus of the British Pharmacological Society.
Jeffrey K. Aronson is a physician and clinical pharmacologist working in the Centre for Evidence-Based Medicine in the Nuffield Department of Primary Care Health Sciences, University of Oxford. He is an Associate Editor of BMJ EBM and a President Emeritus of the British Pharmacological Society.
 Richard Hobbs is a GP and Nuffield Professor of Primary Care Health Sciences, Director, NIHR English School for Primary Care Research and Director, NIHR Applied Research Collaboration (NIHR ARC) Oxford
Richard Hobbs is a GP and Nuffield Professor of Primary Care Health Sciences, Director, NIHR English School for Primary Care Research and Director, NIHR Applied Research Collaboration (NIHR ARC) Oxford
 Kamal R. Mahtani is a GP, Associate Professor and Deputy Director of the Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences, University of Oxford. He is also an Associate Editor at the BMJ Evidence-Based Medicine journal and Director of The Evidence-based Healthcare MSc in Systematic Reviews
Kamal R. Mahtani is a GP, Associate Professor and Deputy Director of the Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences, University of Oxford. He is also an Associate Editor at the BMJ Evidence-Based Medicine journal and Director of The Evidence-based Healthcare MSc in Systematic Reviews
References
1 Fujita T, Sakurai K. Multivariate analysis of risk factors for postoperative pneumonia. The American Journal of Surgery. 1995;169:304–7. doi:10.1016/s0002-9610(99)80163-9
2 Gbinigie OA, Onakpoya IJ, Richards GC, et al. Biomarkers for diagnosing serious bacterial infections in older outpatients: a systematic review. BMC Geriatr 2019;19:190.
3 Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clinical Infectious Diseases. 2007;44:S27–72. doi:10.1086/511159
4 Kawai S, Ochi M, Nakagawa T, et al. Antimicrobial therapy in community-acquired pneumonia among emergency patients in a university hospital in Japan. Journal of Infection and Chemotherapy. 2004;10:352–8. doi:10.1007/s10156-004-0350-2
5 Maruyama T, Gabazza EC, Morser J, et al. Community-acquired pneumonia and nursing home-acquired pneumonia in the very elderly patients. Respiratory Medicine. 2010;104:584–92. doi:10.1016/j.rmed.2009.12.008
6 El-Solh AA, Sikka P, Ramadan F, et al. Etiology of Severe Pneumonia in the Very Elderly. American Journal of Respiratory and Critical Care Medicine. 2001;163:645–51. doi:10.1164/ajrccm.163.3.2005075
7 Gleason PP, Meehan TP, Fine JM, et al. Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia. Arch Intern Med 1999;159:2562–72.
8 Lim WS. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–82. doi:10.1136/thorax.58.5.377
9 Coronavirus Age, Sex, Demographics (COVID-19) – Worldometer. https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/ (accessed 13 Mar 2020).
10 Meehan TP. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA: The Journal of the American Medical Association. 1997;278:2080–4. doi:10.1001/jama.278.23.2080
11 Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med 2006;3:e343.
12 Russell CD, Millar JE, Kenneth Baillie J. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395:473–5. doi:10.1016/s0140-6736(20)30317-2
13 Golda A, Malek N, Dudek B, et al. Infection with human coronavirus NL63 enhances streptococcal adherence to epithelial cells. J Gen Virol. 2011;92(Pt 6):1358–1368. doi:10.1099/vir.0.028381-0
14 WHO guidelines for the global surveillance of severe acute respiratory syndrome (SARS) Updated recommendations October 2004 https://www.who.int/csr/resources/publications/WHO_CDS_CSR_ARO_2004_1.pdf
15 BTS GUIDELINE FOR OXYGEN USE IN ADULTS IN HEALTHCARE AND EMERGENCY SETTINGS British Thoracic Society Emergency Oxygen Guideline Development Group https://www.brit-thoracic.org.uk/document-library/guidelines/emergency-oxygen/bts-guideline-for-oxygen-use-in-healthcare-and-emergency-settings/
16 Metlay JP, Kapoor WN, Fine MJ. Does This Patient Have Community-Acquired Pneumonia? Diagnosing Pneumonia by History and Physical Examination. JAMA. 1997;278(17):1440–1445. doi:10.1001/jama.1997.03550170070035
17 Zhang Y, Fang C, Dong BR, Wu T, Deng JL. Oxygen therapy for pneumonia in adults. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No.: CD006607. DOI: 10.1002/14651858.CD006607.pub4
18 Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet Respiratory Medicine https://doi.org/10.1016/S2213-2600(20)30116-8

